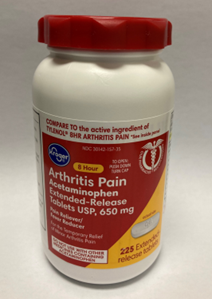
Kroger Arthritis Pain Acetaminophen, 225 count bottles
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 25,660)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Aurobindo Pharma Limited, of Telangana, India
- Reason for Recall:
- The recalled over-the-counter product contains the regulated substance acetaminophen which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The recalled over-the-counter product contains the regulated substance acetaminophen which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
- Remedy:
- Consumers should immediately store the recalled product in a safe location out of reach and sight of children. Contact Kroger for information on how to properly dispose of the product and receive a full refund.
Product Images

Product Information
Full Description:
This recall involves the Kroger brand acetaminophen. The red, white, and yellow label states, Kroger, Acetaminophen, Arthritis Pain, Extended-Release, Tablets USP, 650 mg, 225 extended-release tablets. The bottle has a red continuous thread gear closure. UPC number 041260012846 and lot numbers P2100890, P2100891, P2100992 (each with expiration date Aug-2023) and P2101010 (with expiration date Apr-2023) are included in this recall. The UPC number, lot number and expiration date are printed near the drug facts panel on the label on the back of the bottle.
Product Codes/Lot Numbers:
(About 25,660)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 22164
Related Recalls
Walgreens Pain Reliever Acetaminophen, 150 count bottles
Aurobindo Pharma Limited, of Telangana, India
The recalled over-the-counter products contain the regulated substance acetaminophen which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.