Kroger Aspirin, 300 count bottles and Ibuprofen, 160 count bottles
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 209,430)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Unknown Manufacturer
- Reason for Recall:
- The recalled over-the-counter products contain the regulated substances aspirin and ibuprofen which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The recalled over-the-counter products contain the regulated substances aspirin and ibuprofen which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
- Remedy:
- Consumers should immediately store the recalled products in a safe location out of reach and sight of children. Contact Kroger for information on how to properly dispose of the product and receive a full refund.
Product Images
Product Information
Full Description:
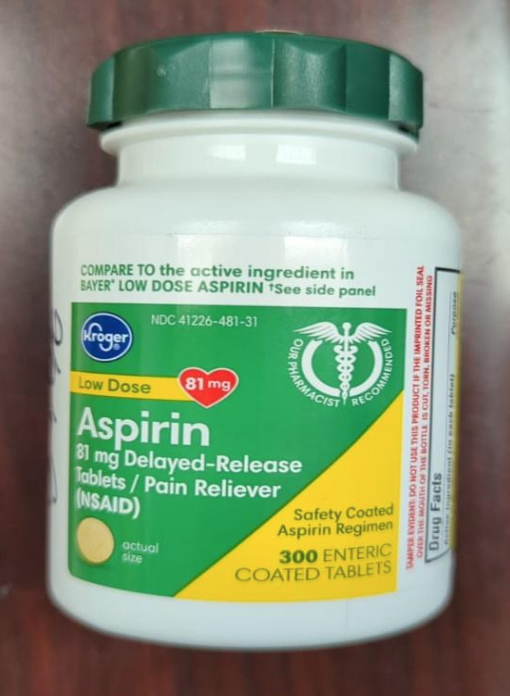
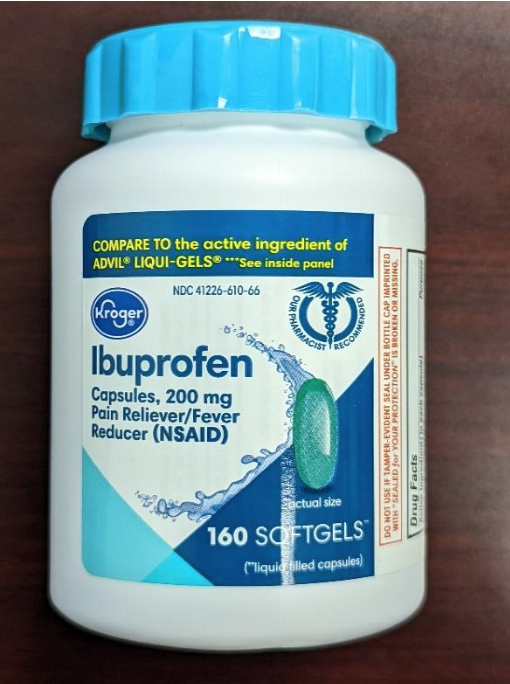
This recall involves the Kroger brand aspirin and ibuprofen over-the-counter drugs. For the aspirin product, the green and yellow label states Kroger, Low Dose, Aspirin, 81 mg Delayed-Release Tablets / Pain Reliever, 300 enteric coated tablets. The bottle has a green continuous thread gear closure. For the ibuprofen product, the blue and white label states Kroger, Ibuprofen, Capsules, 200 mg Pain Reliever / Fever Reducer, 160 softgels. The bottle has a blue continuous thread gear closure. The following UPC and Lot numbers listed in the table are included in this recall and can be found on the label on the back of the bottle. Item UPC Lot numbers Aspirin 4126001295 A077J F032H F035H J011H K031H Ibuprofen 4126001298 FH1163 C11044 C11047 C11064 C11065 C11079 C11084
Product Codes/Lot Numbers:
(About 209,430)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 22160
Related Recalls
AMP MP2 Smart Fitness Machine
Unknown Manufacturer
The arm on the MP2 model of the fitness machine does not lock properly, which can allow the arm to swing unexpectedly, posing a risk of laceration or serious injury.
ANNQUAN Power Strips, Models EX-D112-05 and EX-D106-25
Unknown Manufacturer
The power strips do not contain supplementary overcurrent protection, which creates a risk of fire if the power strips are overloaded. The resulting fire can cause serious injury or death from smoke inhalation and burns.
Blue Wave brand 48-inch and taller above-ground pools
Unknown Manufacturer
The compression strap that surrounds the outside of the pool legs may create a foothold, allowing a child access to the pool, posing a drowning risk.