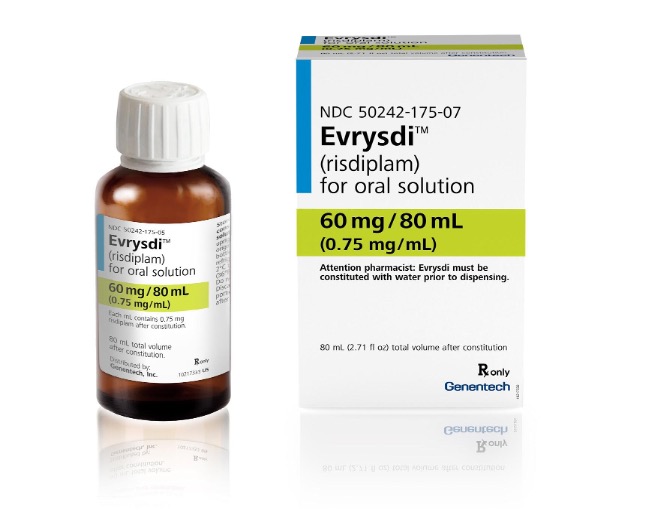
Evrysdi™ (risdiplam)
Class II - Moderate 🏠 Consumer Products
Recalled: March 18, 2021 Genentech, a member of the Roche Group, of South San Francisco, Calif. Children's Products
Nationwide
What Should You Do?
- Check if you have this product: (About 14,000)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Genentech, a member of the Roche Group, of South San Francisco, Calif.
- Reason for Recall:
- The bottles containing the prescription drug can leak due to a fit issue between the press-in bottle adapter and bottleneck. If the bottle is leaking, there is a risk of drug exposure by contact with skin or eyes. Prescription drugs must be in child resistant packaging that prevents children from gaining access to the contents as required by the Poison Prevention Packaging Act (PPPA).
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The bottles containing the prescription drug can leak due to a fit issue between the press-in bottle adapter and bottleneck. If the bottle is leaking, there is a risk of drug exposure by contact with skin or eyes. Prescription drugs must be in child resistant packaging that prevents children from gaining access to the contents as required by the Poison Prevention Packaging Act (PPPA).
- Remedy:
- Consumers should immediately store Evrysdi in a safe location out of reach and sight of children. Consumers should inspect the bottle for any leaking. If it is leaking, contact Genentech for a free replacement. Consumers can continue to use as directed, as the leakage is not anticipated to impact the safety or efficacy of administered medicine. If Evrysdi gets on your skin, wash the area with soap and water. If Evrysdi gets in your eyes, rinse your eyes with water. Genentech is contacting all known purchasers directly.
Product Images

Product Information
Full Description:
This recall involves bottles of the prescription drug Evrysdi, a prescription medicine used to treat spinal muscular atrophy (SMA) in adults and children 2 months of age and older. The recalled 100 mL amber bottles have "Evrysdi (risdiplam) for oral solution," the dosage and "NDC 20242-175-07" on the front of the bottle labels.
Product Codes/Lot Numbers:
(About 14,000)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 21729