Imatinib Mesylate Tablets 100 mg, Imatinib Mesylate Tablets 400 mg, Pregabalin Capsules 50 mg, Pregabalin Capsules 75 mg, Pregabalin Capsules 100 mg, Pregabalin Capsules 150 mg, Sevelamer Carbonate Tablets 800 mg, Tadalafil Tablets 5 mg and Tadalafil Tablets 20 mg
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 21,400)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Dr. Reddy's Laboratories, Ltd., of India
- Reason for Recall:
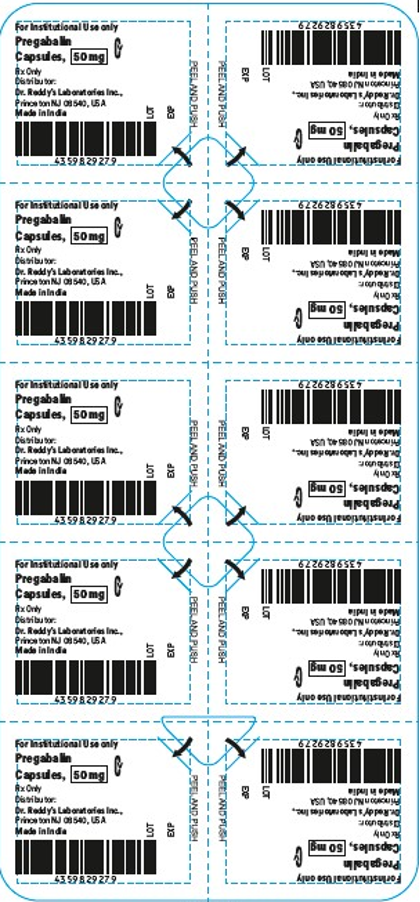
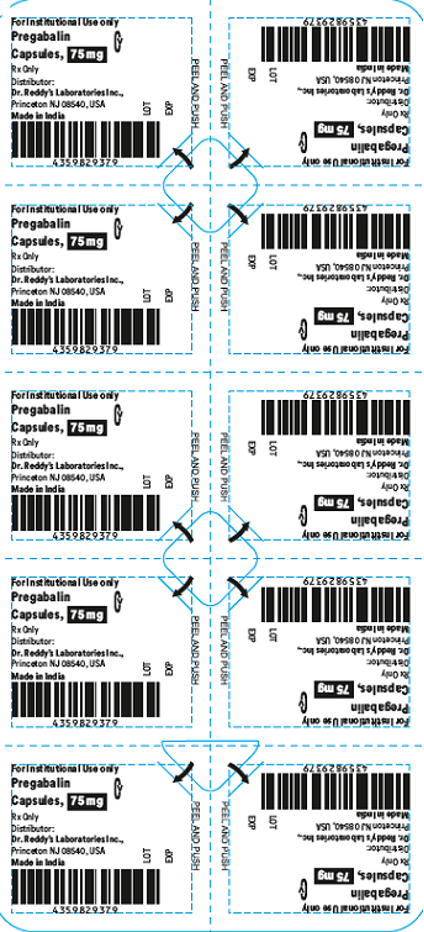
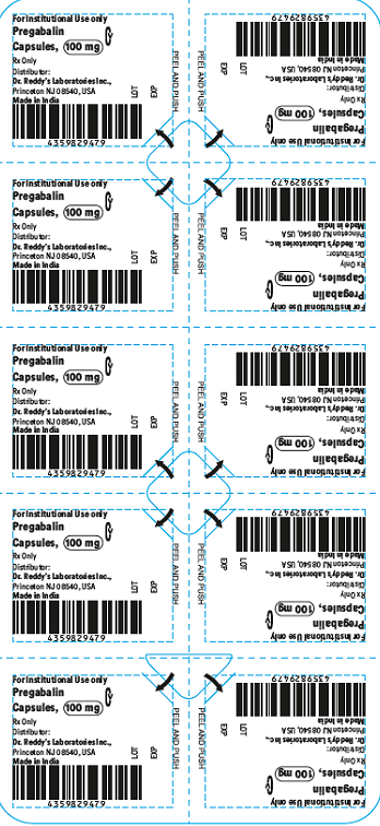
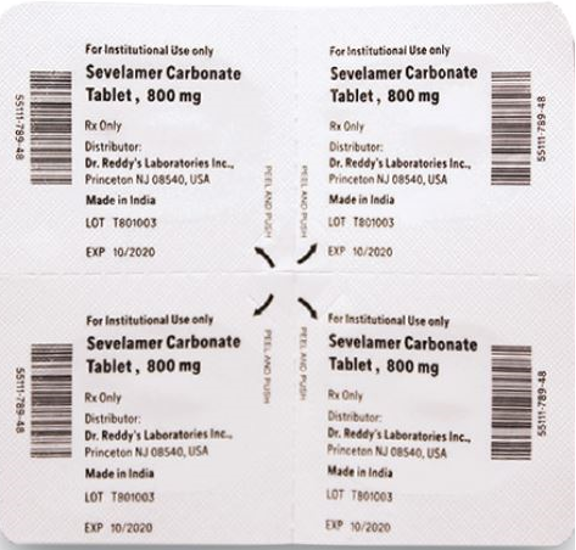
- The products are prescription medications that were labeled and distributed by Dr. Reddy's for institutional use only. The prescription medications were distributed by third party wholesalers to retail pharmacies and could have been dispensed to consumers. The packaging of the products is not child resistant and can pose a risk of poisoning if the contents are swallowed by young children.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The products are prescription medications that were labeled and distributed by Dr. Reddy's for institutional use only. The prescription medications were distributed by third party wholesalers to retail pharmacies and could have been dispensed to consumers. The packaging of the products is not child resistant and can pose a risk of poisoning if the contents are swallowed by young children.
- Remedy:
- Consumers should immediately store the recalled medications in a safe location out of reach of children and contact Dr. Reddy's for a full refund.
Product Images













Product Information
Full Description:
This recall involves blister packages of prescription medications. The name and strength of the medication, "For Institutional Use only," "Rx Only," lot number and expiration date are printed on the outside of the package as well as on the individual blister units. The Dr. Reddy's logo and NDC number are printed on the outside of the package. The recalled medications include the following: Recalled Prescription Drugs NDC Numbers Carton Configurations Lot Numbers Expiration Dates Imatinib Mesylate Tablets 100 mg 43598-344-31 3 blister cards of 10 tablets H2000138 2022-0630 Imatinib Mesylate Tablets 400 mg 43598-345-31 3 blister cards of 10 tablets H2000127 2022-0630 Pregabalin Capsules 50 mg 43598-292-66 5 blister cards of 10 capsules T900876 2021-0630 Pregabalin Capsules 75 mg 43598-293-66 5 blister cards of 10 capsules T901021 2021-0731 Pregabalin Capsules 100 mg 43598-294-66 5 blister cards of 10 capsules T901022 2021-0731 Pregabalin Capsules 150 mg 43598-295-66 5 blister cards of 10 capsules T901023 2021-0731 Sevelamer Carbonate Tablets 800 mg 55111-789-11 4 blister cards of 25 tablets T801003, T000009, T900221 2020-1031, 2021-1231, 2021-0228 Tadalafil Tablets 5 mg 43598-575-31 3 blister cards of 10 tablets T000376 2022-0131 Tadalafil Tablets 20 mg 43598-573-31 3 blister cards of 10 tablets T000425 2022-0228
Product Codes/Lot Numbers:
(About 21,400)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 21089