WHILL Personal Electric Vehicles, Model Ci
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 1,160)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- WHILL, Inc. (Japan), of Japan
- Reason for Recall:
- The vehicle's control pad can malfunction causing the power to turn on/off and the speed to increase/decrease, posing crash and injury hazards.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The vehicle's control pad can malfunction causing the power to turn on/off and the speed to increase/decrease, posing crash and injury hazards.
- Remedy:
- Consumers should immediately stop using the recalled personal mobility vehicle and contact WHILL for a free replacement control pad.
Product Images


Product Information
Full Description:
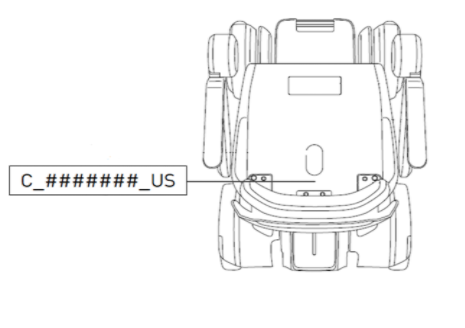
This recall involves WHILL Model Ci. The product is a personal mobility device that is powered by a lithium ion battery, is occupant-controlled and motorized. The serial number of the recalled device ranges from C_1711188_US to C_1909011_US. The serial number label is located on the seat assembly. WHILL and Model Ci are printed on the battery compartment. The vehicles have one chair with a platform and four wheels. The chairs are black and the arm covers come in white, red, blue and black colors.
Product Codes/Lot Numbers:
(About 1,160)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 20177