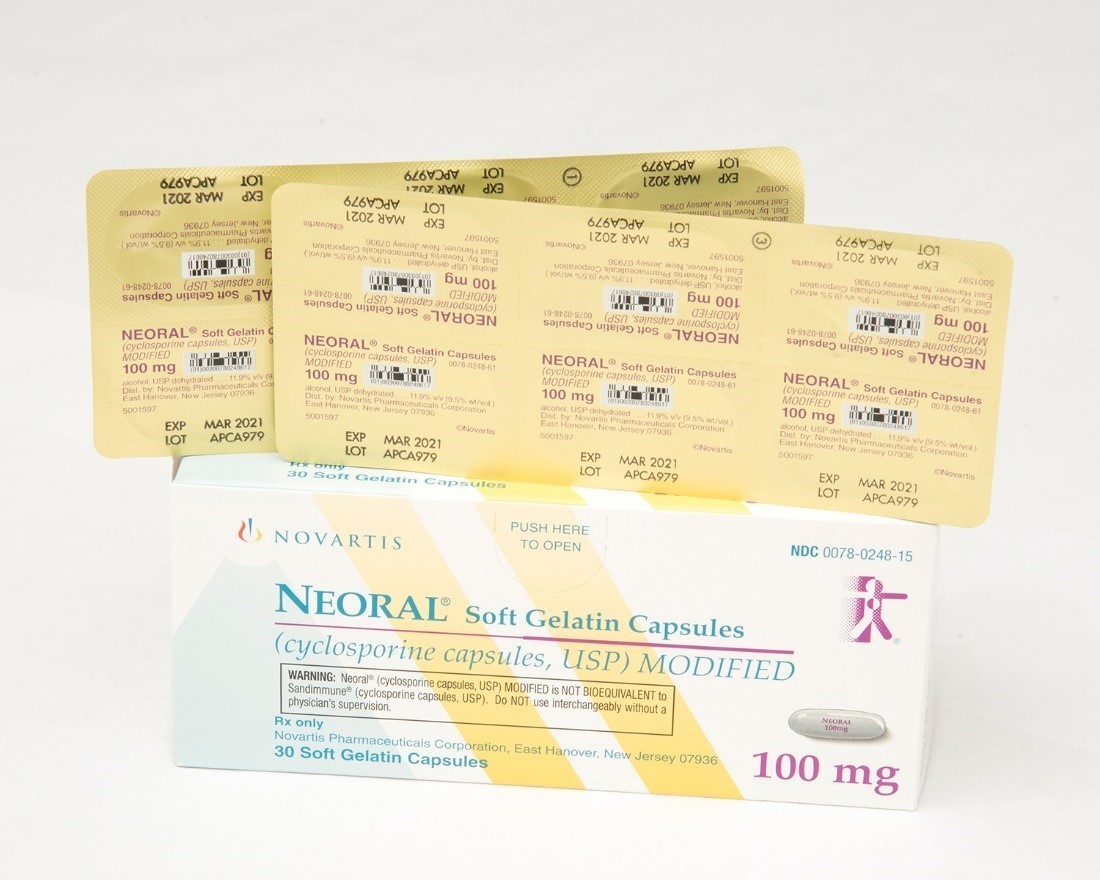
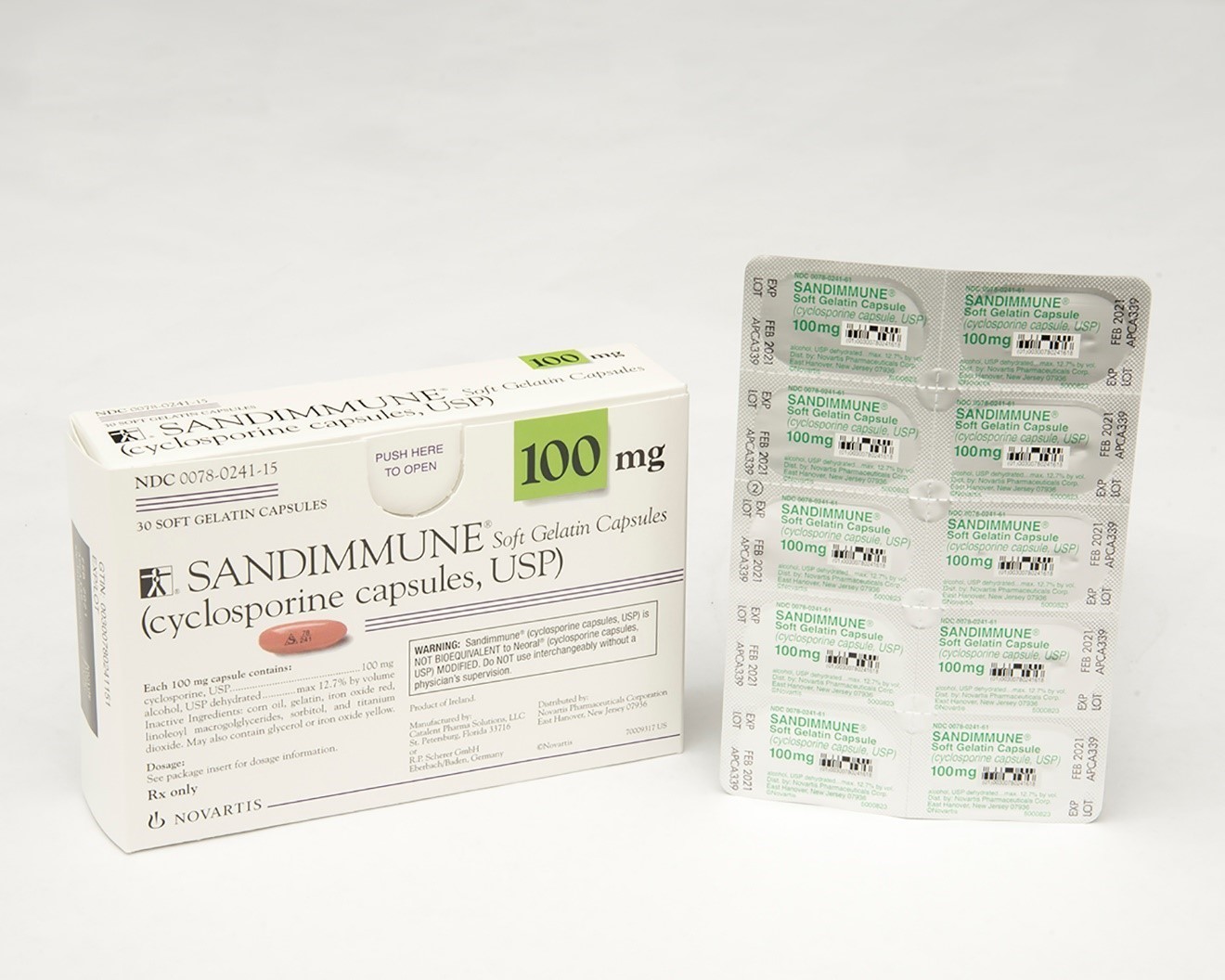
Sandimmune® (cyclosporine capsules, USP) 100 mg soft gelatin capsules and Neoral® (cyclosporine capsules, USP) MODIFIED 100 mg soft gelatin capsules prescription drug blister packages
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 73,000)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Unknown Manufacturer
- Reason for Recall:
- The prescription drug packaging is not child resistant as required by the Poison Prevention Packaging Act (PPPA), posing a risk of poisoning if the contents are swallowed by young children.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The prescription drug packaging is not child resistant as required by the Poison Prevention Packaging Act (PPPA), posing a risk of poisoning if the contents are swallowed by young children.
- Remedy:
- Consumers should immediately secure the product out of the sight and reach of children and contact the firm to request a free child-resistant pouch in which to store the blister package medications. Consumers can continue to use the medication as directed. The child-resistant pouches should be used to store these medications until new child-resistant blister packaging is available.
Product Images
Product Information
Full Description:
This recall involves blister packages of prescription medications Sandimmune® (cyclosporine capsules, USP) 100 mg soft gelatin capsules and Neoral® (cyclosporine capsules, USP) MODIFIED 100 mg soft gelatin capsules from Novartis. Packages of Sandimmune 100 mg contain three blister cards with ten soft gelatin capsules per card and packages of Neoral 100 mg contain five blister cards with six soft gelatin capsules per card. The recalled blister packages have "Novartis," the name of the medication, dosage, NDC, lot number and expiration date on the outer package and on the blister cards. Only 100 mg doses of these medications with the following NDC and lot numbers and expiration dates are included in this recall: Recalled Prescription Drugs NDC Numbers Lot Numbers Expiration Date Sandimmune® (cyclosporine capsules, USP) 100 mg soft gelatin capsules 0078-0241-15 0078-0241-61 APCA136 APCA339 APCA793 APCC238 09/2020 02/2021 01/2022 07/2022 Neoral® (cyclosporine capsules, USP) MODIFIED 100 mg soft gelatin capsules 0078-0248-15 0078-0248-61 APCA437 APCA979 07/2020 03/2021
Product Codes/Lot Numbers:
(About 73,000)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 20091
Related Recalls
AMP MP2 Smart Fitness Machine
Unknown Manufacturer
The arm on the MP2 model of the fitness machine does not lock properly, which can allow the arm to swing unexpectedly, posing a risk of laceration or serious injury.
ANNQUAN Power Strips, Models EX-D112-05 and EX-D106-25
Unknown Manufacturer
The power strips do not contain supplementary overcurrent protection, which creates a risk of fire if the power strips are overloaded. The resulting fire can cause serious injury or death from smoke inhalation and burns.
Blue Wave brand 48-inch and taller above-ground pools
Unknown Manufacturer
The compression strap that surrounds the outside of the pool legs may create a foothold, allowing a child access to the pool, posing a drowning risk.