Home elevators
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 680 (in addition, about 1700 were sold in Canada))
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Cambridge Elevating, of Cambridge, Ontario Canada
- Reason for Recall:
- The landing door on the elevators can unlock without the elevator present, posing a fall hazard.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The landing door on the elevators can unlock without the elevator present, posing a fall hazard.
- Remedy:
- Consumers should immediately stop using the recalled elevators and contact the firm to be directed to a local certified technician for a free repair.
Product Images



Product Information
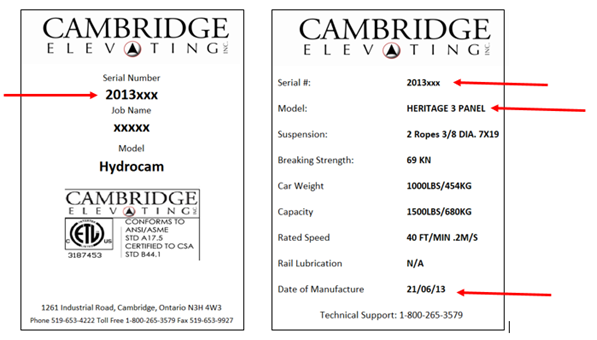
Full Description:
This recall involves Cambridge Elevating Elmira, Heritage & Hybrid model home elevators using the first and second generation micro-controller systems (BES 1 & BES 2). "Cambridge Elevating" is printed on the label inside the elevators. The elevators have an operating panel inside the cab with a digital display, a stop button, the elevator capacity and a phone box. Only elevators with production date codes 1/2/2009 through 29/11/17 printed on the manufacture label inside the elevator wall are included in the recall.
Product Codes/Lot Numbers:
(About 680 (in addition, about 1700 were sold in Canada))
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 19091