StairMaster® branded 8G Gauntlet Stepmill machines
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 3,500)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
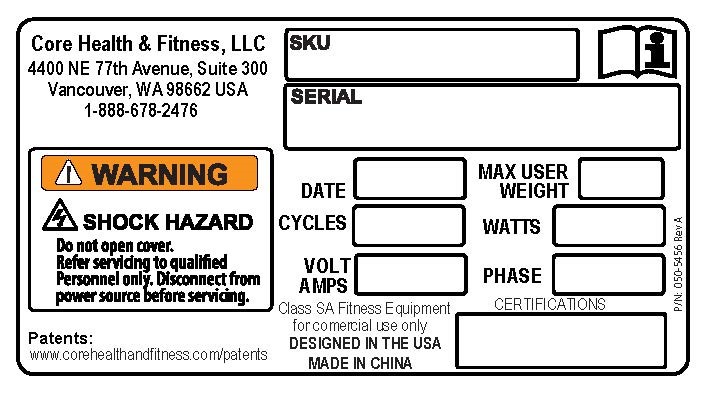
- Land America Health and Fitness, of Xiamen, Xingling, China
- Reason for Recall:
- The steps can accelerate rapidly without input from the user, posing a fall hazard.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The steps can accelerate rapidly without input from the user, posing a fall hazard.
- Remedy:
- Consumers should immediately stop using the recalled exercise machines and contact Core Health & Fitness for a free repair. The firm is contacting all known purchasers directly.
Product Images


Product Information
Full Description:
This recall involves StairMaster branded 8G Gauntlet Stepmill machines with model numbers 9-5250 & 9-5270. The recalled machines were sold in red, black and red/yellow. The recalled machines consist of a revolving staircase and a backlit LCD console. The model number is printed on the base of the unit. The date code is in the YYWW format (1816 means year 2018, week 16). The date code is the section of the serial number at digits 8 - 11 (see photo). The date code range for SKU 9-5250 is from 1734 to 1823. The date code range for SKU 9-5270 is from 1813 to 1816. Stairmaster is printed on both sides of the unit and on the shrouds.
Product Codes/Lot Numbers:
(About 3,500)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 18764