Well at Walgreens pain and itch relief cream
Class II - Moderate 🏠 Consumer Products
Recalled: January 30, 2018 Natureplex LLC, of Olive Branch, Mass. Other Consumer Products
Nationwide
What Should You Do?
- Check if you have this product: (About 74,000)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Natureplex LLC, of Olive Branch, Mass.
- Reason for Recall:
- The packaging is not child resistant as required by the Poison Prevention Packaging Act. The pain and itch relief cream contains lidocaine, posing a risk of poisoning to young children.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The packaging is not child resistant as required by the Poison Prevention Packaging Act. The pain and itch relief cream contains lidocaine, posing a risk of poisoning to young children.
- Remedy:
- Consumers should immediately place the recalled pain and itch relief cream out of the reach of children and return it to Walgreens for a full refund.
Product Images
Product Information
Full Description:
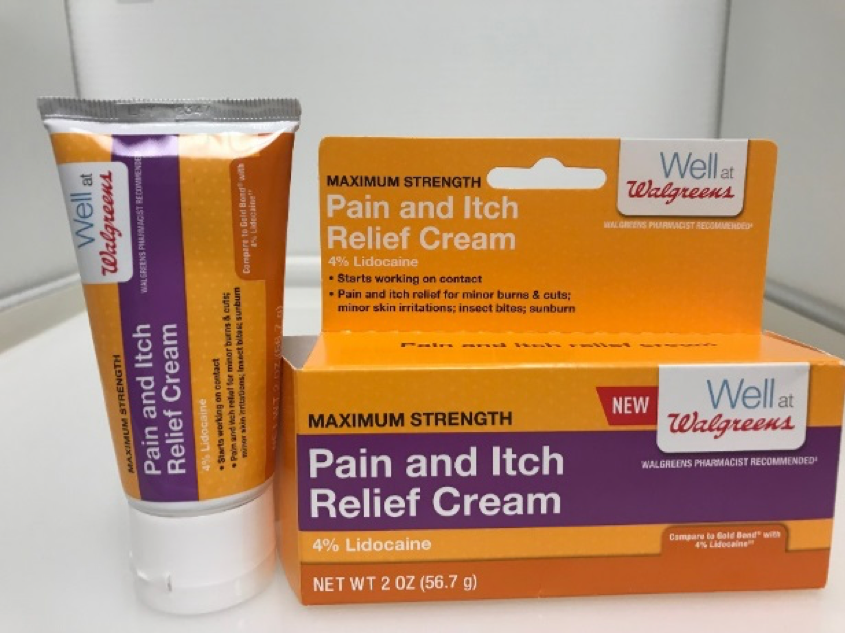
The recalled Well at Walgreens Pain and Itch Relief Cream tube and packaging are orange with a purple stripe with "Maximum Strength," "Pain and Itch Relief Cream 4% Lidocaine" and "NET WT 2 OZ (56.7 grams)" printed in white on the front. The Well at Walgreens logo is located on the front upper right corner. The packaging contains the UPC bar code 3 11917 18962 8 on the back.
Product Codes/Lot Numbers:
(About 74,000)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 18086