Zolpidem Tartrate Sublingual tablets
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 5,700 boxes of 30 blister packs)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Novel Laboratories, of Somerset, N.J.
- Reason for Recall:
- The packaging for the prescription drug is not child-resistant as required by the Poison Prevention Packaging Act, posing a risk of poisoning if swallowed by children.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The packaging for the prescription drug is not child-resistant as required by the Poison Prevention Packaging Act, posing a risk of poisoning if swallowed by children.
- Remedy:
- Consumers should immediately stop using the recalled tablets and contact Novel Laboratories for instructions to receive a full refund.
Product Images



Product Information
Full Description:
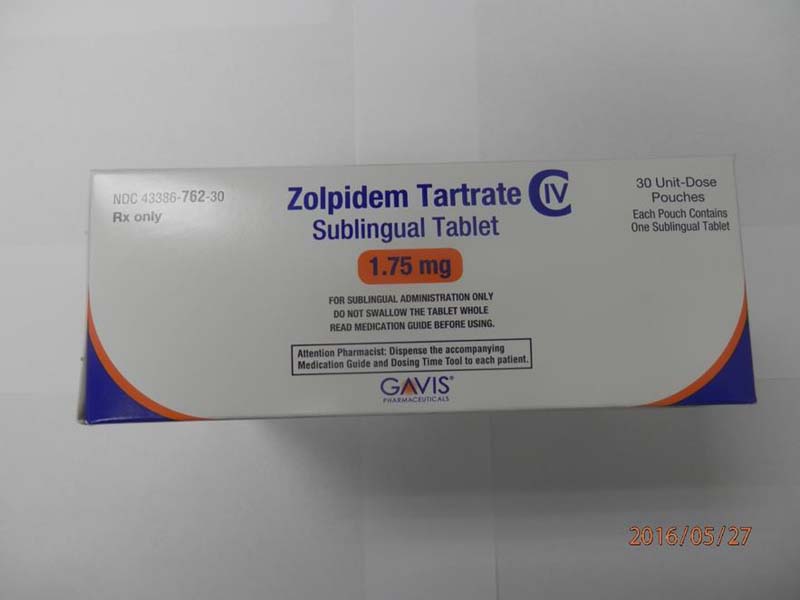
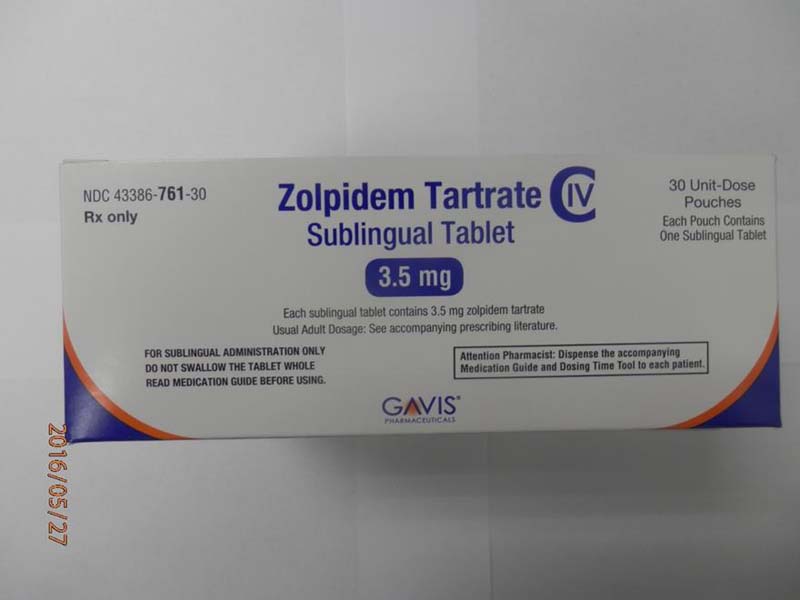
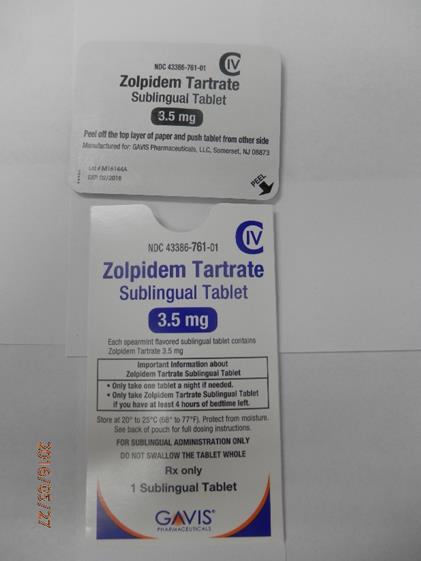
This recall involves 1.75 mg and 3.5 mg Zolpidem Tartrate sublingual (rapid dissolve) tablets from Novel Laboratories. The prescription medication is a sleep drug. The packaging for the 1.75 mg tablets has lot number M16140A, 1.75 mg and national drug code (NDC) 43386-762-30. The packaging for the 3.5 mg tablets has lot number M16144A, 3.5mg and national drug code (NDC) 43386-761-30. The Zolpidem Tartrate is packaged as a single tablet in a peel-and-push blister pack inside an outer open-ended pouch with 30 pouches per box. The lot number and an expiration date of 02/2018 are printed on the bottom left of the pouches. The NDC is printed on the top left corner of the boxes. "Gavis" is printed in blue on the center of the white boxes and pouches.
Product Codes/Lot Numbers:
(About 5,700 boxes of 30 blister packs)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 16232