Scope® Original Mint Mouthwash, 1-Liter Size
Class I - DangerousWhat Should You Do?
- Check if you have this product: (About 35,000 bottles)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Seek medical attention if needed: If you've consumed this product and feel unwell, contact your doctor immediately.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
⚠️ Emergency: If you experience severe symptoms after consuming this product, call 911 or Poison Control at 1-800-222-1222.
Recall Details
- Company:
- The Procter & Gamble Co., of Cincinnati, Ohio
- Reason for Recall:
- The mouthwash contains ethyl alcohol and certain bottles have malfunctioning child-resistant closures. Ethyl alcohol is toxic and can cause serious injury or death if ingested by children.
- Classification:
- Class I - Dangerous
Dangerous or defective products that predictably could cause serious health problems or death.
- Status:
- ongoing
- Hazard:
- The mouthwash contains ethyl alcohol and certain bottles have malfunctioning child-resistant closures. Ethyl alcohol is toxic and can cause serious injury or death if ingested by children.
- Remedy:
- Consumers should keep this product out of the reach of children. Consumers who purchased the product with the expectation that it would be in child-resistant packaging can contact Procter & Gamble for a full refund or a replacement coupon. Adult consumers can continue to use the product as directed.
Product Images

Product Information
Full Description:
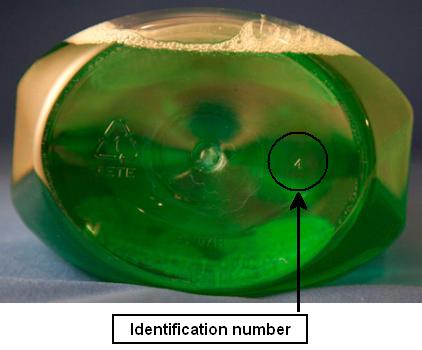
This recall involves some bottles of Scope® Original Mint Mouthwash in 1-liter size. The recalled bottles have the number 4 on the bottom of the bottle. The bottles with the 4 on the bottom may not be child-resistant. Consumers can also attempt to twist the cap open. If the cap can be twisted off without squeezing the tabs on the cap, the package is not child-resistant.
Product Codes/Lot Numbers:
(About 35,000 bottles)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 10280
Related Recalls
Vicks Dayquil Cold & Flu 24-Count Bonus Pack Liquicaps
The Procter & Gamble Co., of Cincinnati, Ohio
The cold and flu medicine contains acetaminophen and is not in child-resistant packaging and lacks the statement, "This Package for Households Without Young Children," as required by the Poison Prevention Packaging Act. This medicine could cause serious health problems or death to a child if several of the capsules are swallowed.