Contessa Office Chairs
Class II - ModerateWhat Should You Do?
- Check if you have this product: (About 60,000)
- Do not eat it: Even if it looks and smells fine, do not consume this product.
- Throw it away or return it: You can return the product to the store for a full refund.
- Report problems: Report any issues to the FDA's Safety Reporting Portal.
Recall Details
- Company:
- Okamura Corp., of Japan
- Reason for Recall:
- The bolts holding the seat to the frame can loosen and separate, posing a fall hazard to consumers.
- Classification:
- Class II - Moderate
Products that might cause a temporary health problem, or pose a slight threat of a serious nature.
- Status:
- ongoing
- Hazard:
- The bolts holding the seat to the frame can loosen and separate, posing a fall hazard to consumers.
- Remedy:
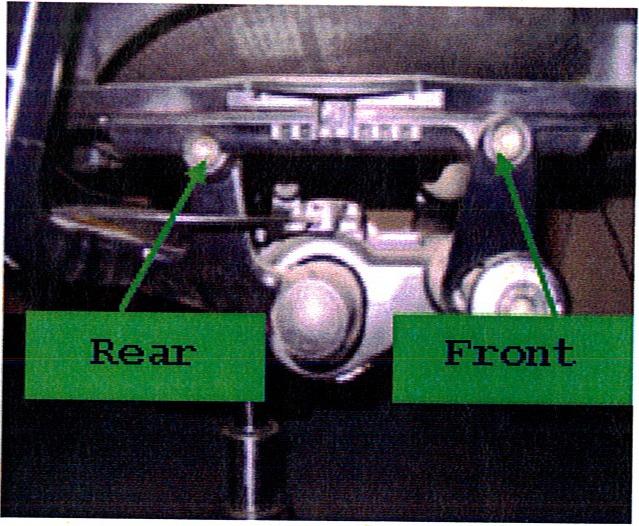
- Consumers should inspect the chair by checking the four bolts that attach the seat bottom to the chair frame. If any of the four bolts is loose or missing, consumers should stop using the chair immediately and contact a Teknion dealer for a free repair.
Product Images

Product Information
Full Description:
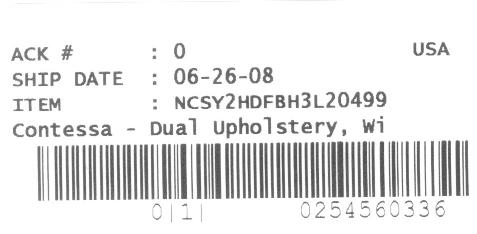
This recall involves all Contessa chairs shipped prior to June 30, 2008. Each Contessa chair has a label indicating the date of shipment (see picture), which is located on the bottom of the chair seat.
Product Codes/Lot Numbers:
(About 60,000)
Official Source
Always verify recall information with the official CPSC source:
View on CPSC.govCPSC Recall Number: 09716